Intravascular Lithotripsy Holds Promise 2:1 Versus Absorbable Stents In PAD
Vascular surgeons and interventional radiologists, showed twice the level of enthusiasm for the innovation offered by Intravascular Lithotripsy than the next technology believed to show most promise, absorbable stents, in offering innovation in Peripheral Arterial Disease PAD treatment over the next three years.
FOR IMMEDIATE RELEASE.
Vascular surgeons and interventional radiologists, show twice the level of enthusiasm for the opportunities offered by Intravascular Lithotripsy (IVL) than the next technology believed to show most promise, absorbable stents, in offering innovation in Peripheral Arterial Disease (PAD) treatment over the next three years.
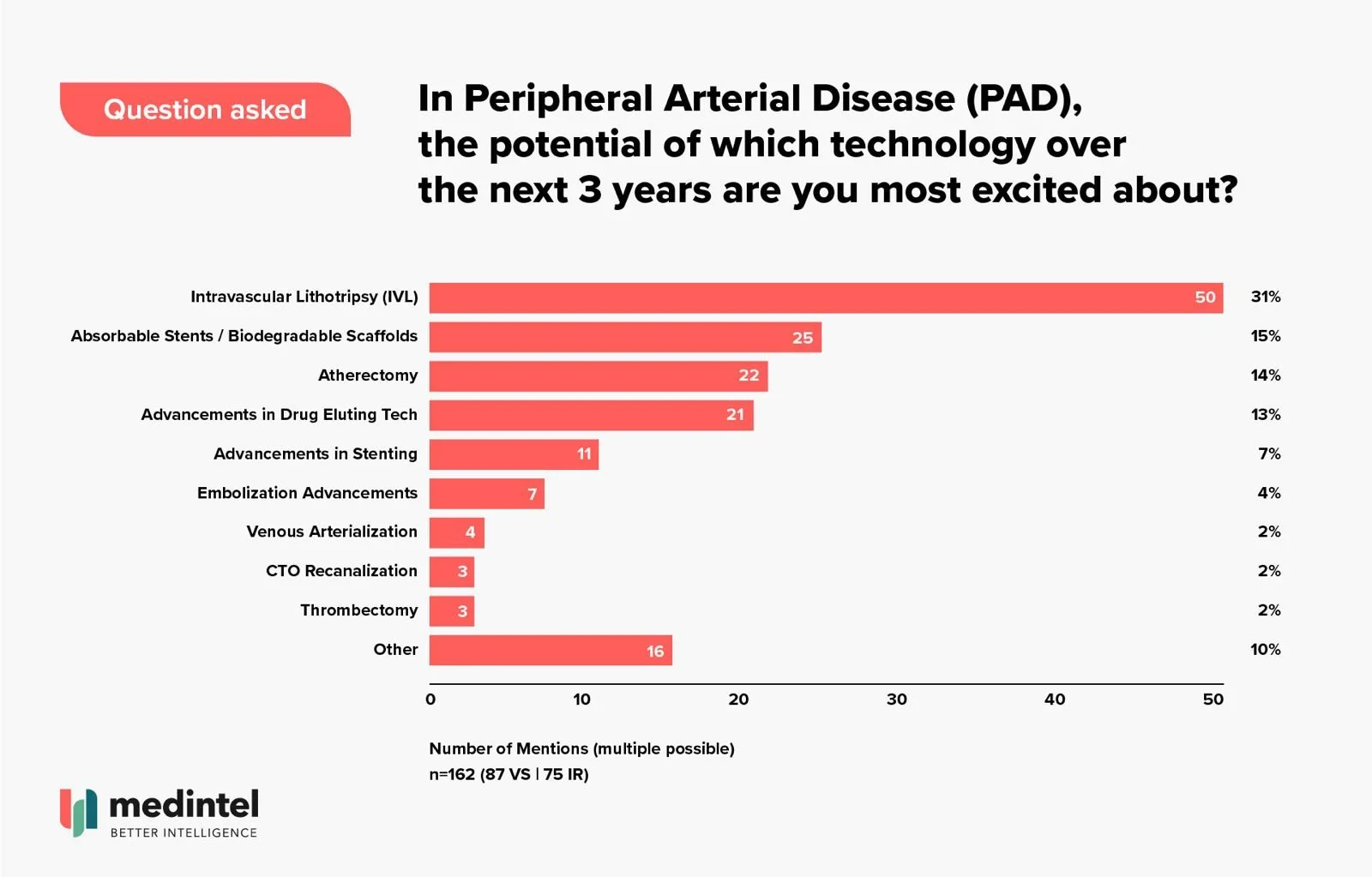
medintel surveyed 162 physicians (interventional radiologists, vascular surgeons) from across the EU and US, and 31% of physicians named IVL as the technology they're most excited about over the next three years.
This represents more than double the interest in the next category, absorbable stents/biodegradable scaffolds at 15%. Similar differences were seen with only 14% of physicians excited about the possibilities for atherectomy and the same for drug eluting technology at 13%.
Technology physicians are most excited about the potential of in PAD. Surveyed in September 2025.
Intravascular Lithotripsy technology is rapidly transforming the treatment landscape for Peripheral Arterial Disease, with significant growth opportunities forecast. Tibial vessels are highlighted as the highest potential growth area for IVL technology as new, lower-profile devices with longer balloons enter the market.
IVL has revolutionised calcium management in vascular procedures. Physicians report IVL is dramatically reducing the need for stenting in femoral-popliteal treatments while improving safety profiles compared to traditional atherectomy.
‘IVL has transformed calcium management over the last few years,’ notes Kilian Toal, Director at medintel. ‘The ease of use, short learning curve, and applicability across most vessels mean it is rapidly becoming the first choice for many physicians in preparation for severe calcific disease.’
IVL is viewed by physicians as especially beneficial when treating diabetic and end-stage renal disease patients, populations projected to grow significantly in coming years.
While Shockwave Medical currently dominates the IVL market, competitive devices from companies including Bolt Medical (recently acquired by Boston Scientific), Abbott (through CSI acquisition), Amplitude Vascular Systems (AVS) and FastWave Medical are in development or early clinical trials.
Market competition is expected to intensify as reimbursement policies catch up with clinical practice and the growing evidence base continues to demonstrate IVL's effectiveness.
ENDS.
About the Research
Methodology Note: This analysis is based on a survey of 162 (US and EU interventional radiologists, vascular surgeons) conducted in September 2025. This survey represents a focused snapshot of early adopter perspectives rather than a comprehensive market study.
medintel is a specialist medical market research consultancy. For more information, contact intel@medintel.co.uk.
medintel has the capability to conduct larger-scale quantitative and qualitative research across broader physician populations. If you're interested in more extensive RDN market research or custom studies, please get in touch.
Renal Denervation Market Poised for Mainstream Adoption by 2030, New Physician Survey Reveals
New medintel survey: 75% of interventional cardiologists predict renal denervation will become standard hypertension treatment by 2030
FOR IMMEDIATE RELEASE
75% of practicing interventional cardiologists predict procedure will become standard hypertension treatment despite current limited adoption
[October 31st 2025] - A new market research study by medintel reveals that renal denervation (RDN) is experiencing a remarkable resurgence, with three-quarters of interventional cardiologists practicing the procedure predicting the procedure will become mainstream hypertension treatment by 2030.
The survey of 123 interventional cardiologists across the United States and Europe found that while only 37% currently perform RDN procedures, 75% of those with hands-on experience believe the technology will achieve widespread adoption within five years.
"After nearly a decade in the wilderness following early clinical setbacks, renal denervation is back with rigorous evidence and renewed industry commitment," said Kilian Toal, Partner, at medintel. "The physician confidence we're seeing represents a fundamental shift in how the medical community views this technology."
Ends.
Background Notes:
Market Revival Built on Solid Foundation
The RDN market has transformed dramatically since its early boom-and-bust cycle. Initial enthusiasm in the 2010s led to major acquisitions, including Medtronic's $800 million purchase of Ardian. However, the field went quiet after Medtronic's pivotal Symplicity HTN-3 trial failed in 2014.
The current revival is anchored by FDA approvals in November 2023 for both ReCor Medical's Paradise ultrasound system and Medtronic's Symplicity Spyral radiofrequency system—the first renal denervation devices approved in the United States. Boston Scientific's recent $540 million acquisition of SoniVie signals renewed industry investment.
Technology and Patient Selection Trends Emerge
This research survey on renal denervation practices revealed clear preferences among practicing physicians:
80% prefer radiofrequency-based systems over ultrasound technology
68% identify resistant hypertension patients (on ≥3 medications) as the ideal candidates
US and European adoption rates are remarkably similar at 36% and 39% respectively
Barriers Remain But Solutions in Sight
Despite optimism, physicians identified key obstacles to broader adoption of renal denervation:
Access and reimbursement coverage (55% of respondents)
Patient identification and responder selection (52%)
Cost and hospital economics (48%)
Lack of long-term clinical evidence (43%)
Regional differences emerged, with US physicians more concerned about long-term evidence and economics, while European colleagues focused on patient selection and awareness.
Looking Ahead
The study suggests RDN's path to mainstream status depends on addressing systemic challenges around reimbursement, patient selection tools, and long-term evidence. A pending Medicare national coverage decision could serve as a pivotal catalyst for broader US adoption.
About the Research
Methodology Note: This analysis is based on a survey of 123 (US n=67 and EU n=56) interventional cardiologists conducted in August 2025. This survey represents a focused snapshot of early adopter perspectives rather than a comprehensive market study.
medintel is a specialist medical market research consultancy. For more information, contact intel@medintel.co.uk.
medintel has the capability to conduct larger-scale quantitative and qualitative research across broader physician populations. If you're interested in more extensive RDN market research or custom studies, please get in touch.